Change Parts Washing Machine
Change Parts Washing Machine for Validated cGMP Cleaning
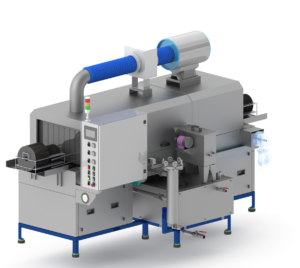
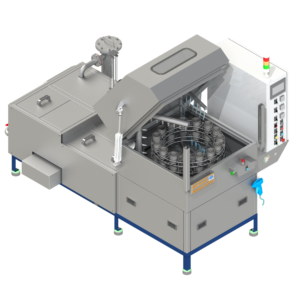
A change parts washing machine is a cGMP‑grade automatic washer designed to clean product‑contact change parts from tablet presses, capsule fillers, cartoners, blister machines and liquid filling lines. By standardising and validating the cleaning process, it helps prevent cross‑contamination and carryover between products and batches.
Configured as a compact pharmaceutical parts washer, it automates wash, rinse and drying of nozzles, star wheels, dosing discs, tooling and other change parts, reducing manual labour and improving cleaning consistency.
What is a Change Parts Washing Machine?
A change parts washing machine (also called a contact‑part or format‑part washer) is a specialised cGMP washer for parts that directly touch the product. Parts are loaded onto racks or manifolds, the chamber door is closed, and programmable cycles run with controlled time, temperature, flow and detergent dosing.
Unlike generic washers, these cGMP washers are built with a hygienic design, pharmaceutical‑grade materials and documentation to support IQ/OQ/PQ and routine cleaning validation.
cGMP Washers for Pharma Change Parts
Modern cGMP washers for change parts offer:
- Hygienic 316L stainless steel chambers and piping with sloped surfaces for complete drainability.
- Flexible racks and injection manifolds to wash internal pathways of pumps, valves, hoses and tooling.
- PLC/HMI control with recipe management, audit trails and, when needed, 21 CFR Part 11‑compliant data handling.
This ensures the pharmaceutical parts washer delivers repeatable, traceable cleaning cycles that meet regulatory expectations and internal SOPs.
Pharmaceutical Glass Washer and Parts Washer Combined
In many facilities, the same unit serves both as a pharmaceutical glass washer and a change‑parts washer, handling glass bottles, vials, beakers and labware alongside stainless steel parts and accessories.
With custom racks and injection systems, the change parts washing machine can wash:
- Filling line change parts and format parts.
- Glassware and containers used in production or QC labs.
- Trays, scoops, funnels and other small equipment.
This combined approach reduces equipment count and simplifies validation and maintenance.
Change Parts Washing Machine – Typical Specifications
Adjust values to your actual designs:
Specification | Value (example) | Notes |
Machine type | cGMP change parts washing machine | Compact cabinet or rack design |
Chamber configuration | Single‑door (through‑the‑wall optional) | For production or wash area |
Load capacity | 100–300 kg per cycle | Racks for change parts and glassware |
Process phases | Pre‑wash / Wash / Rinse / Final rinse / Dry | Recipe‑controlled |
Water types | PW / WFI / tap (for pre‑wash) | Configurable by phase |
Spray system | Oscillating arms + injection manifolds | Internal and external coverage |
Temperature range | Ambient to 90 °C | Controlled and recorded |
Drying | HEPA‑filtered hot air | In‑chamber drying |
Controls | PLC + HMI, recipe and alarm logs | 21 CFR Part 11 capable |
Construction | 316L stainless steel, sanitary design | Fully drainable, cGMP compliant |
Validation support | IQ/OQ/PQ protocols, FAT/SAT documentation | For regulated sites |
Benefits of an Automatic Change Parts Washer
Implementing an automatic change parts washing machine offers key benefits for pharma manufacturers:
- Regulatory compliance: cGMP design, validated cycles and complete records support inspections and audits.
- Shorter changeovers: Parts washing time is predictable and can be planned into batch scheduling, reducing downtime between products.
- Reduced manual cleaning risks: Automated pharmaceutical parts washers minimise operator variability, reduce exposure to cleaning agents and free technicians for higher‑value tasks.
Frequently Asked Questions
What parts are cleaned in a change parts washing machine?
A change parts washing machine is used to clean product‑contact parts such as dosing discs, nozzles, star wheels, chutes, feed screws, guides, tooling, pump bodies and small accessories from tablet, capsule, filling and packaging lines. Many systems also function as a pharmaceutical glass washer for compatible glassware.
How do cGMP washers help with cleaning validation?
cGMP washers provide controlled, repeatable cycles with recorded parameters (time, temperature, flow, conductivity, etc.), plus documentation and support for IQ/OQ/PQ. This allows cleaning processes to be developed, challenged and validated according to regulatory and internal validation guidance
Can one machine handle multiple product families and lines?
Yes. A change parts washing machine typically stores multiple recipes and can be equipped with different racks and manifolds for various part sets. With proper segregation and validated cycles, the same pharmaceutical parts washer can support several product lines while maintaining control of cross‑contamination risk.
What utilities are required for a change parts washer?
Depending on model and configuration, a change parts washing machine typically requires purified water (and possibly WFI), drain, electrical power (and/or steam), and ventilation for heat and moisture. cGMP models may also require integration with building management or data systems for monitoring and audit trails.
How is this different from a standard washing machine or domestic washer?
A pharmaceutical washer for change parts is designed specifically for regulated environments: hygienic design, pharmaceutical‑grade materials, automated cycle control, and full documentation. Domestic or non‑cGMP industrial washers do not provide the validation, traceability or hygienic construction needed for pharmaceutical contact‑part cleaning

 hydrojetuhpl@gmail.com |
hydrojetuhpl@gmail.com |